LIMITATIONS OF CURRENT THERAPEUTIC OPTIONS
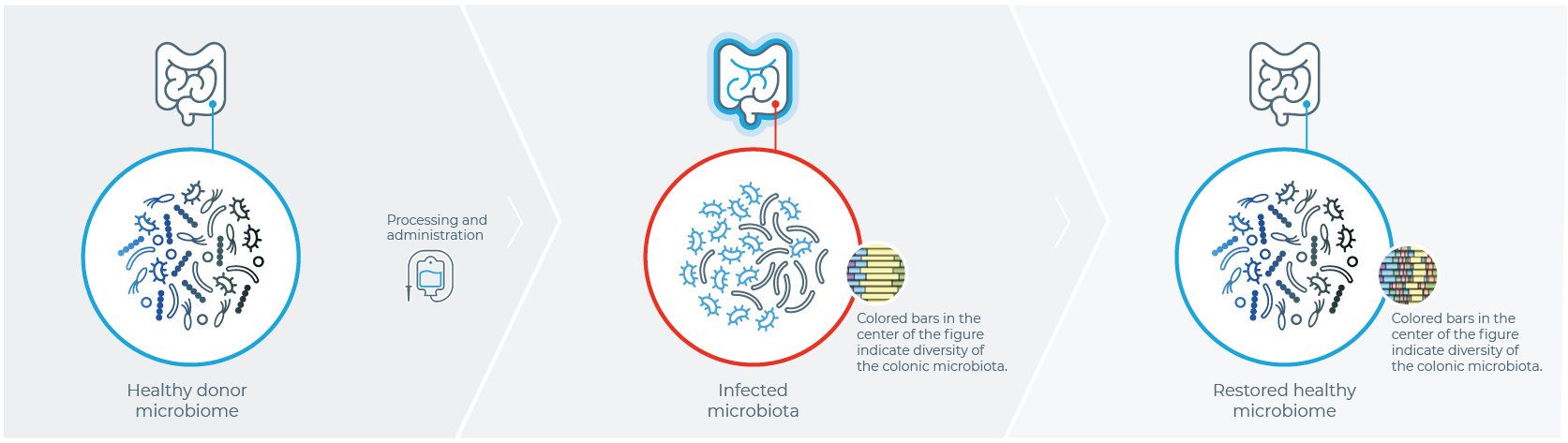
ONE HISTORIC APPROACH TO MICROBIOME RESTORATION
IS FECAL MICROBIOTA TRANSPLANTATION (FMT)1
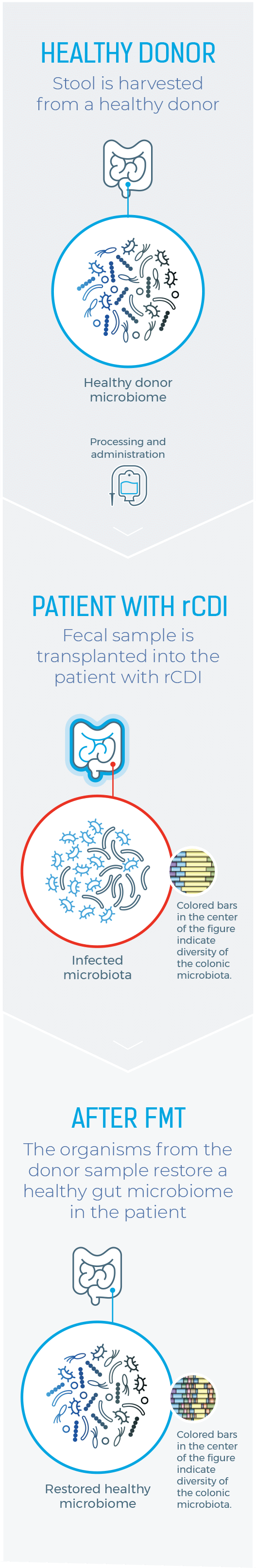
Healthy Donor
Stool is harvested from a healthy donor
PATIENT WITH rCDI
Fecal sample is transplanted into the patient with rCDI
After FMT
The organisms from the donor sample restore a healthy gut microbiome in the patient
References: 1. American Gastroenterological Association. https//www.gastro.org/practice-guidance/gi-patient-center/topic/fecal-microbiota-transplantation-fmt. Accessed August 31, 2021. 2. Tariq R, et al. Clin Infect Dis. 2019;68(8):1351-1358. 3. Bafeta A, et al. Ann Intern Med. 2017;167(1)34-39. 4. Joseph J, et al. Clin Transi Sci. 2019;12(3)206-208. 5. Guh AY, et al. N Engl J Med. 2020;382(14):1320-1330. 6. McDonald LC, et al. Clin Infect Dis. 2018;66:el-e48.
Limitations of Current Therapeutic Options
SEVERAL LIMITATIONS ARE FOUND ACROSS CLINICAL STUDIES,
DIAGNOSIS, AND TREATMENT
THERE IS VARIABILITY ACROSS CLINICAL TRIALS
Cure rates were lower in randomized controlled trials than in open-label studies (67.7% vs 82.7%, respectively; P<.001).2
This inconsistency is due to considerable heterogeneity among randomized controlled trials, with marked differences in study structure, control groups, fecal transplant materials, and outcome assessments.2,3
LACK OF
STANDARDIZATION
Likewise, the lack of product standardization and administration methods has created a situation where a regulated, safe, and effective product is critically needed.4
PATIENTS ENROLLED IN CLINICAL TRIALS MAY NOT BE A TRUE REFLECTION OF PATIENTS SEEN IN THE REAL WORLD
Strict inclusion and exclusion criteria in randomized controlled trials lead to inclusion of a small portion of patients from daily clinical practice, limiting generalizability of results to patients seen in clinical practice.2
CDI TESTING IS INCONSISTENT IN CLINICAL PRACTICE
There is no gold standard: sensitivity and specificity of current toxin tests for CDI are highly variable. Moreover, in the US, there is no consensus on best diagnostic testing for CDI.5,6
OUR
COMMITMENT
Ferring is committed to exploring the potential of a microbiome-based therapeutic that is studied in a comprehensive population reflective of routine clinical practice for the treatment of recurrent C. difficile infection.
References: 1. American Gastroenterological Association. https//www.gastro.org/practice-guidance/gi-patient-center/topic/fecal-microbiota-transplantation-fmt. Accessed August 31, 2021. 2. Tariq R, et al. Clin Infect Dis. 2019;68(8):1351-1358. 3. Bafeta A, et al. Ann Intern Med. 2017;167(1)34-39. 4. Joseph J, et al. Clin Transi Sci. 2019;12(3)206-208. 5. Guh AY, et al. N Engl J Med. 2020;382(14):1320-1330. 6. McDonald LC, et al. Clin Infect Dis. 2018;66:el-e48.
RESTORE THE GUT MICROBIOME
RESTORE HOPE IN PATIENTS WITH RECURRENT C. diff INFECTION
Further research is essential to ensure availability of a safe, effective, and standardized microbiota-based therapeutic that can help—along with antibiotic treatment—restore the microbiome and break the vicious cycle of recurrent C. diff infection.








References: 1. American Gastroenterological Association. https//www.gastro.org/practice-guidance/gi-patient-center/topic/fecal-microbiota-transplantation-fmt. Accessed August 31, 2021. 2. Tariq R, et al. Clin Infect Dis. 2019;68(8):1351-1358. 3. Bafeta A, et al. Ann Intern Med. 2017;167(1)34-39. 4. Joseph J, et al. Clin Transi Sci. 2019;12(3)206-208. 5. Guh AY, et al. N Engl J Med. 2020;382(14):1320-1330. 6. McDonald LC, et al. Clin Infect Dis. 2018;66:el-e48.