MICROBIAL VOLUME AND DIVERSITY
THE GUT MICROBIOME IS COMPOSED OF HIGHLY DIVERSE MICROORGANISMS LIVING IN THE HUMAN INTESTINAL TRACT
Considered a distinct and essential organ within the human body, the gut microbiome contains 100 trillion microorganisms and consists of an estimated 500-1000 different species.1-4

Common bacterial phyla in the gut1,5:
- Bacteroidetes
- Firmicutes
- Proteobacteria
- Actinobacteria
- Fusobacteria
- Verrucomicrobia
Comprise ~90% of microbiota
References: 1. Thursby E, Juge N. Biochem J. 2017;474(11):1823-1836. 2. Gilbert JA, et al. Nat Med. 2018;24(4):392-400. 3. Antharam VC, et al. J Clin Microbiol. 2013;51(9):2884-2892. 4. Marchesi JR, et al. Gut. 2016;65(2):330-339. 5. Jandhyala SM, et al. World J Gastroenterol. 2015;21(29):8787-8803. 6. Wexler AG, Goodman AL. Nat Microbiol. 2017;2(1):17115. 7. Rinninella E, et al. Microorganisms. 2019;7(1):14. 8. Gill SR, et al. Science. 2006;312(5778):1355-1359. 9. Kurokawa K, et al. DNA Res. 2007;14(4):169-181. 10. Li X, et al. Biomed Pharmacother. 2021;137:111290. 11. Kho ZY, Lal SK. Front Microbiol. 2018;9(1835):1-23. 12. Sokol H, et al. Proc Natl Acad Sci USA. 2008;105(43):16731-16736. 13. Willing BP, et al. Gastroenterology. 2010;139(6):1844-1854.e1. 14. Machiels K, et al. Gut. 2014;63(8):1275-1283. 15. Paust S, et al. Proc Natl Acad Sci USA. 2004;101(28):10398-10403. 16. El Aidy S, et al. Mucosal Immunol. 2012;5(5):567-579. 17. Lawley TD, Walker AW. Immunology. 2013;138(1):1-11. 18. Herrera G, et al. Sci Rep. 2021;11(1):10849. 19. Theriot CM, Young VB. Annu Rev Microbiol. 2015;69:445-461.
MICROBIAL VOLUME AND DIVERSITY
BACTEROIDETES AND FIRMICUTES ARE MOST PREVALENT IN THE GUT MICROBIOME AND WORK SYMBIOTICALLY6-8

Gram-negative Bacteroidetes
Their ability to adapt and persist in changing gut environments allows abundance and stability,6,9-11 providing long-term associations with human hosts and enabling functions that include6..
- Immunomodulatory effects6,a
- Inhibitory activities against C. diff and reduction of colonization12,a,b

Gram-positive Firmicutes
Composed of helpful and harmful bacteria,3,13 Firmicutes are the most abundant and diverse bacterial gut species,3 with functions that include:
- Anti-inflammatory effects14-16,c
- Fortification of gut barrier (along with other bacteria)17-19,b
aSome Bacteroidetes. bIn preclinical studies. cDemonstrated in Crohn's disease, inflammatory bowel disease, and ulcerative colitis studies.
Deficiencies in
Bacteroidetes and
Firmicutes are
particularly
associated with
C. diff infection.3,8,20
References: 1. Thursby E, Juge N. Biochem J. 2017;474(11):1823-1836. 2. Gilbert JA, et al. Nat Med. 2018;24(4):392-400. 3. Antharam VC, et al. J Clin Microbiol. 2013;51(9):2884-2892. 4. Marchesi JR, et al. Gut. 2016;65(2):330-339. 5. Jandhyala SM, et al. World J Gastroenterol. 2015;21(29):8787-8803. 6. Wexler AG, Goodman AL. Nat Microbiol. 2017;2(1):17115. 7. Rinninella E, et al. Microorganisms. 2019;7(1):14. 8. Gill SR, et al. Science. 2006;312(5778):1355-1359. 9. Kurokawa K, et al. DNA Res. 2007;14(4):169-181. 10. Li X, et al. Biomed Pharmacother. 2021;137:111290. 11. Kho ZY, Lal SK. Front Microbiol. 2018;9(1835):1-23. 12. Sokol H, et al. Proc Natl Acad Sci USA. 2008;105(43):16731-16736. 13. Willing BP, et al. Gastroenterology. 2010;139(6):1844-1854.e1. 14. Machiels K, et al. Gut. 2014;63(8):1275-1283. 15. Paust S, et al. Proc Natl Acad Sci USA. 2004;101(28):10398-10403. 16. El Aidy S, et al. Mucosal Immunol. 2012;5(5):567-579. 17. Lawley TD, Walker AW. Immunology. 2013;138(1):1-11. 18. Herrera G, et al. Sci Rep. 2021;11(1):10849. 19. Theriot CM, Young VB. Annu Rev Microbiol. 2015;69:445-461.
MICROBIAL VOLUME AND DIVERSITY
THE BROAD CONSORTIUM OF BACTERIA WITHIN THE GUT MICROBIOME FUNCTIONS TO PROMOTE OVERALL HEALTH
Aids in health digestive function19

Protects from harmful microorganisms and plays a role in immunity19
Acts as a barrier and achieves colonization resistance1,19
References: 1. Thursby E, Juge N. Biochem J. 2017;474(11):1823-1836. 2. Gilbert JA, et al. Nat Med. 2018;24(4):392-400. 3. Antharam VC, et al. J Clin Microbiol. 2013;51(9):2884-2892. 4. Marchesi JR, et al. Gut. 2016;65(2):330-339. 5. Jandhyala SM, et al. World J Gastroenterol. 2015;21(29):8787-8803. 6. Wexler AG, Goodman AL. Nat Microbiol. 2017;2(1):17115. 7. Rinninella E, et al. Microorganisms. 2019;7(1):14. 8. Gill SR, et al. Science. 2006;312(5778):1355-1359. 9. Kurokawa K, et al. DNA Res. 2007;14(4):169-181. 10. Li X, et al. Biomed Pharmacother. 2021;137:111290. 11. Kho ZY, Lal SK. Front Microbiol. 2018;9(1835):1-23. 12. Sokol H, et al. Proc Natl Acad Sci USA. 2008;105(43):16731-16736. 13. Willing BP, et al. Gastroenterology. 2010;139(6):1844-1854.e1. 14. Machiels K, et al. Gut. 2014;63(8):1275-1283. 15. Paust S, et al. Proc Natl Acad Sci USA. 2004;101(28):10398-10403. 16. El Aidy S, et al. Mucosal Immunol. 2012;5(5):567-579. 17. Lawley TD, Walker AW. Immunology. 2013;138(1):1-11. 18. Herrera G, et al. Sci Rep. 2021;11(1):10849. 19. Theriot CM, Young VB. Annu Rev Microbiol. 2015;69:445-461.
DYSBIOSIS AND C. diff Infection
DYSBIOSIS IS MOST OFTEN CAUSED BY ENVIRONMENTAL FACTORS, INCLUDING ANTIBIOTICS1
HOMEOSTASIS

During homeostasis, the microbiota is composed of diverse organisms known to benefit host health
STRESS DIET HYGIENE USE OF ANTIMICROBIALS

DYSBIOSIS
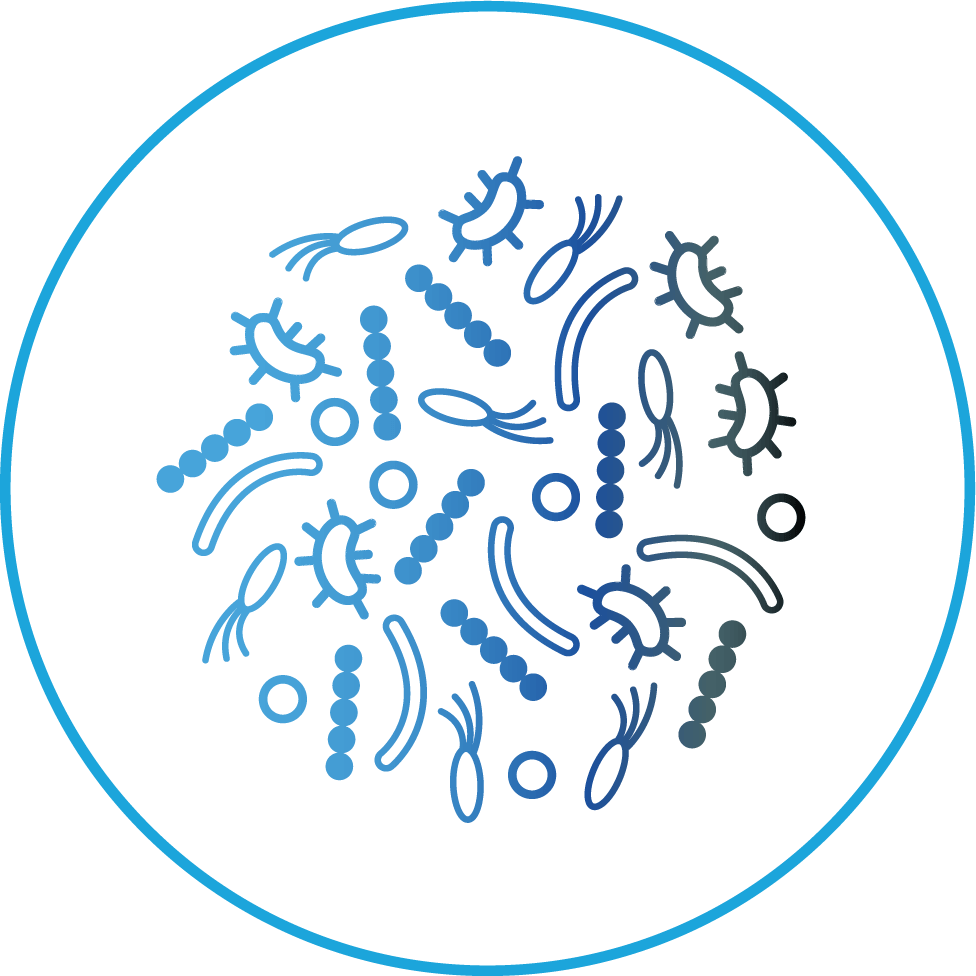 Pathobiont
Pathobiont expansion
 Reduced
Reduced diversity
 Loss of beneficial microbes
Loss of beneficial microbesDysbiosis is the disruption of the
composition and/or diversity of the
gut microbiome
References: 1. Petersen C, Round JL. Cellular Microbial. 2014;16(7):1024-1033. 2. Bien J, et al. Ther Adv Gastroenterol. 2013;6(1):53-68. 3. Staley C, et al. Arch Med Res. 2017;48(8):766-773.
DYSBIOSIS AND C. diff Infection
DISRUPTION OF THE GUT MICROBIOME LEADS TO AN ENVIRONMENT SUITED FOR THE PROLIFERATION OF CLOSTRIDIOIDES DIFFICILE 2,3
The gut microbiota generally play a role in colonization resistance by which the native organisms prevent pathogenic microbes from flourishing.2,3
When the relationship between the gut and its healthy flora becomes imbalanced, dysbiosis results...leading to an intestinal microenvironment susceptible to pathogenic insult from opportunistic bacteria, such as C. diff—capable of causing a wide spectrum of symptoms ranging from mild diarrhea to sepsis.2
References: 1. Petersen C, Round JL. Cellular Microbial. 2014;16(7):1024-1033. 2. Bien J, et al. Ther Adv Gastroenterol. 2013;6(1):53-68. 3. Staley C, et al. Arch Med Res. 2017;48(8):766-773.
C. diff Infection: An Urgent Threat
CLOSTRIDIOIDES DIFFICILE INFECTION DECLARED AN URGENT PUBLIC HEALTH THREAT1
C. diff emerged as an important pathogen, following the introduction of broad-spectrum antibiotics2
An increase in severe cases of CDI was noted in Canada, the United States, and Europe, which was attributed to the emergence of epidemic types of C. difficile3
Hypervirulent NAP1/B1/027 strain emerges in North America and Europe4
Reported that CDI eclipsed methicillin-resistant Staphylococcus aureus (MRSA) as the leading hospital onset healthcare-facility-related infection5,6
CDC identified CDI as an URGENT PUBLIC HEALTH THREAT1,a
CDC = US Centers for Disease Control and Prevention.
aCDC defines urgent threats as those that may not be currently widespread but have the potential to become so and require urgent public health attention to identify infections and to limit transmission.1
References: 1. Centers for Disease Control and Prevention. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed August 31, 2021. 2. Kelly CP. Clin Microbiol Infect. 2012;18(suppl 6):21-27. 3. Smits WK, et al. Nat Rev Dis Primers 2016;2:16020. 4. Fatima R, Aziz M. Cureus. 2019;11(1):e3977. 5. Lessa FC, et al. N Engl J Med. 2015;372(9):825-834. 6. Miller BA, et al. Infect Control Hosp Epidemiol. 2011;32(4):387-390.
CLINICAL BURDEN OF C. DIFF INFECTION
A VICIOUS CYCLE OF RECURRENCE WITH A SIGNIFICANT BURDEN1,2
Significant complications associated with CDI
Recurrence:
Up to 35%3,4,a
Subsequent Recurrence:
Up to 60%5-9
Sepsis:
27%6
Colectomy:
7%10,b
Heart Failure:
43%9
Mortality:
6%3
Mortality in patients with COVID-19 and CDI: 44%8

RECURRENT C. DIFF INFECTION BURDEN
RECURRENT CDI (RCDI) AFFECTS PATIENT QUALITY OF LIFE, HOSPITAL QUALITY METRICS, AND REIMBURSEMENT
-
 Up to 85% of all patients with rCDI were hospitalized in 12 months12
Up to 85% of all patients with rCDI were hospitalized in 12 months12 -
 57% of patients with at least one CDI recurrence experienced ≥2 admissions within 12 months9
57% of patients with at least one CDI recurrence experienced ≥2 admissions within 12 months9 -
 Hospitalizations average 18 days for patients with rCDI9
Hospitalizations average 18 days for patients with rCDI9 -
 84% of patients with rCDI are readmitted12
84% of patients with rCDI are readmitted12 -
 −$3K to −$29K gap exists in reimbursement per patient13
−$3K to −$29K gap exists in reimbursement per patient13 -
 $131K to $207K comprised total cost of a patient with rCDI13
$131K to $207K comprised total cost of a patient with rCDI13
PHYSICAL/PSYCHOLOGICAL RECURRENT C. DIFF INFECTION BURDEN
THE PHYSICAL AND PSYCHOLOGICAL IMPACT OF RECURRENT CDI IS HIGH15
In an observational, cross-sectional study surveying 350 self-reported patients with CDI15

~41%
believed they would never get rid of post-CDI symptoms
Used with permission from Creative Commons. https://creativecommons.org/licenses/by/4.0/legalcode.15
aWithin 8 weeks after initial C. diff infection diagnosis.
bFirst recurrence.
References: 1. Centers for Disease Control and Prevention. https://www.cdc.gov/drugresistance/pdf/threats-report/clostridioides-difficile-508.pdf. Accessed August 25, 2021. 2. Feuerstadt P, et al. J Med Econ. 2020;23(6):603-609. 3. Lessa FC, et al. N Engl J Med. 2015;372(9):825-834. 4. Cornely OA, et al. Clin Infect Dis. 2012;55(suppl 2):s154-s161. 5. Leong C, Zelenitsky S. Can J Hosp Pharm. 2013;66(6):361-368. 6. Kelly CP. Clin Microbiol Infect. 2012;18(suppl 6):21-27. 7. Riddle DJ, et al. Infect Dis Clin North Am. 2009;23(3):727-743. 8. Smits WK, et al. Nat Rev Dis Primers. 2016;2:16020. 9. Nelson WW, et al. J Manag Care Spec Pharm. 2021;27(7):828-838. 10. Feuerstadt P, et al. SAGE Open Med. 2021;9:2050312120986733. 11. Sandhu A, et al. Emerg Infect Dis. 2020;26(9):2272-2274. 12. Rodrigues R, et al. Infect Control Hosp Epidemiol. 2017;38(2):196-202. 13. McDonald LC, et al. Clin Infect Dis. 2018;66(7):e1-e48. 14. Zilberberg MD, et al. Medicine. 2018;97(36):e12212. doi:10.1097/MD.0000000000012212. 15. Lurienne L, et al. J Patient Rep Outcomes. 2020;4(1):14.
STANDARD OF CARE MAY BE INCOMPLETE
THE STANDARD OF CARE FOR CDI IS ALSO A PREDOMINANT RISK FACTOR FOR RECURRENCE1-3
Up to 35% of patients with CDI will experience recurrence within 8 weeks after initial CDI diagnosis.4,5
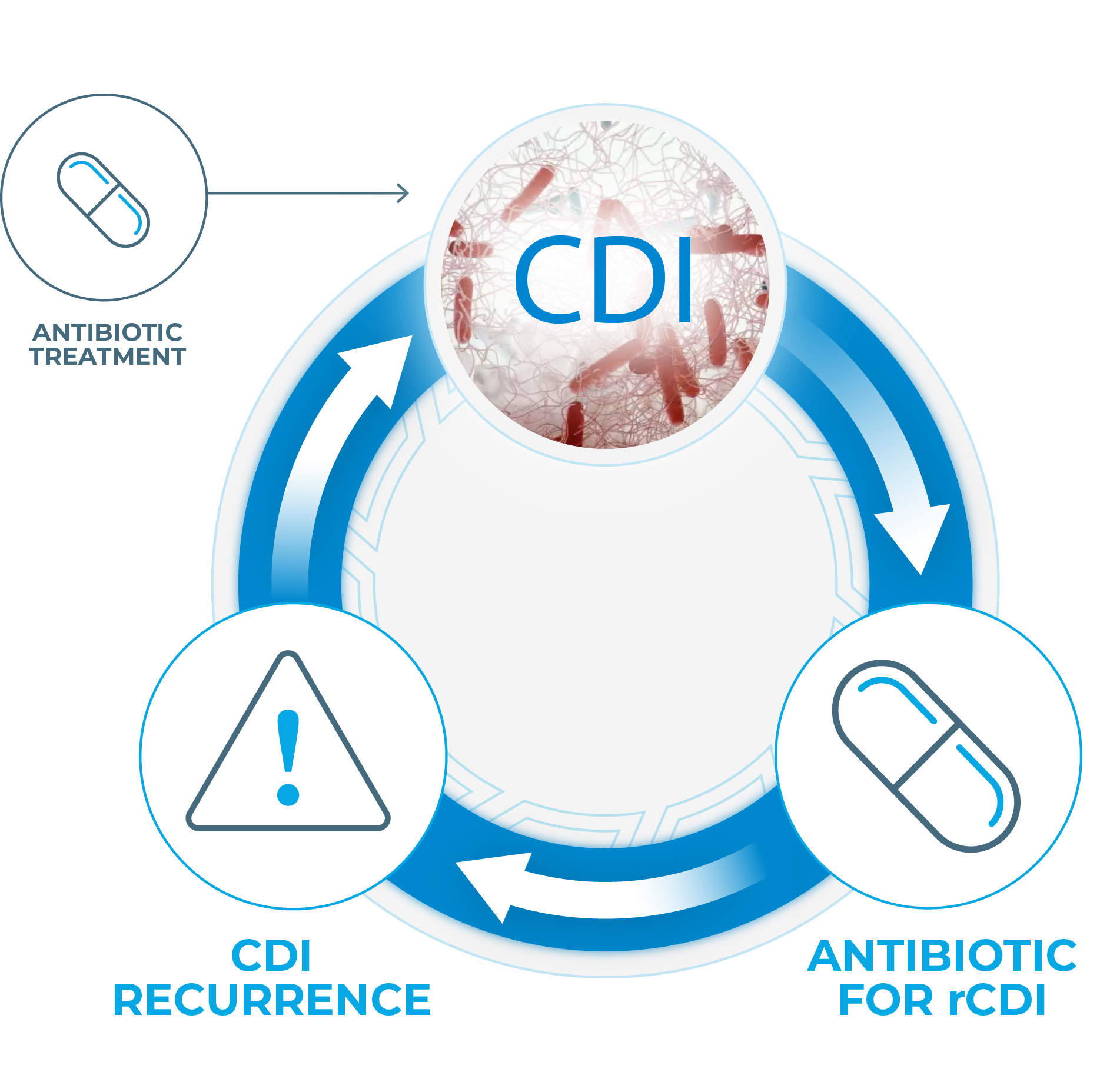
Antibiotic treatment is commonly associated with high rates of rCDI because it6
- Does not address the
underlying dysbiosis - Has a deleterious impact
on the microbiota - Negatively impacts colonization resistance
References: 1. DePestel DD, et al. J Pharm Pract. 2013;26(5):464-475. 2. Knight CL, Surawicz CM. Med Clin North Am. 2013;97(4):523-536. 3. Aukes L, et al. Open Forum Infect Dis. 2021;8(3):ofab052. 4. Lessa FC, et al. N Engl J Med. 2015;372(9):825-834. 5. Cornely OA, et al. Clin Infect Dis. 2012;55(suppl 2):s154-s161. 6. Antharam VC, et al. J Clin Microbiol. 2013;51(9):2884-2892. 7. Fitzpatrick F, et al. Clin Microbiol Infect. 2012;18(suppl 6):2-4. 8. van Nood E, et al. N Engl J Med. 2013;368(5):407-415.
STANDARD OF CARE MAY BE INCOMPLETE
RESTORING A HEALTHY GUT MICROBI0ME IS ESSENTIAL
Thus starts a cycle of C. diff infection and reinfection—impeding microbiome recovery, exacerbating morbidity, and creating a substantial economic burden.7
References: 1. DePestel DD, et al. J Pharm Pract. 2013;26(5):464-475. 2. Knight CL, Surawicz CM. Med Clin North Am. 2013;97(4):523-536. 3. Aukes L, et al. Open Forum Infect Dis. 2021;8(3):ofab052. 4. Lessa FC, et al. N Engl J Med. 2015;372(9):825-834. 5. Cornely OA, et al. Clin Infect Dis. 2012;55(suppl 2):s154-s161. 6. Antharam VC, et al. J Clin Microbiol. 2013;51(9):2884-2892. 7. Fitzpatrick F, et al. Clin Microbiol Infect. 2012;18(suppl 6):2-4. 8. van Nood E, et al. N Engl J Med. 2013;368(5):407-415.
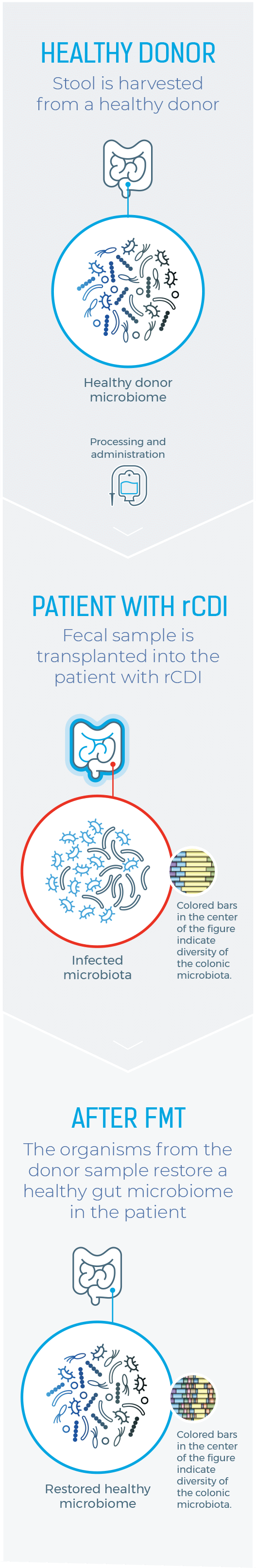
LIMITATIONS OF CURRENT THERAPEUTIC OPTIONS
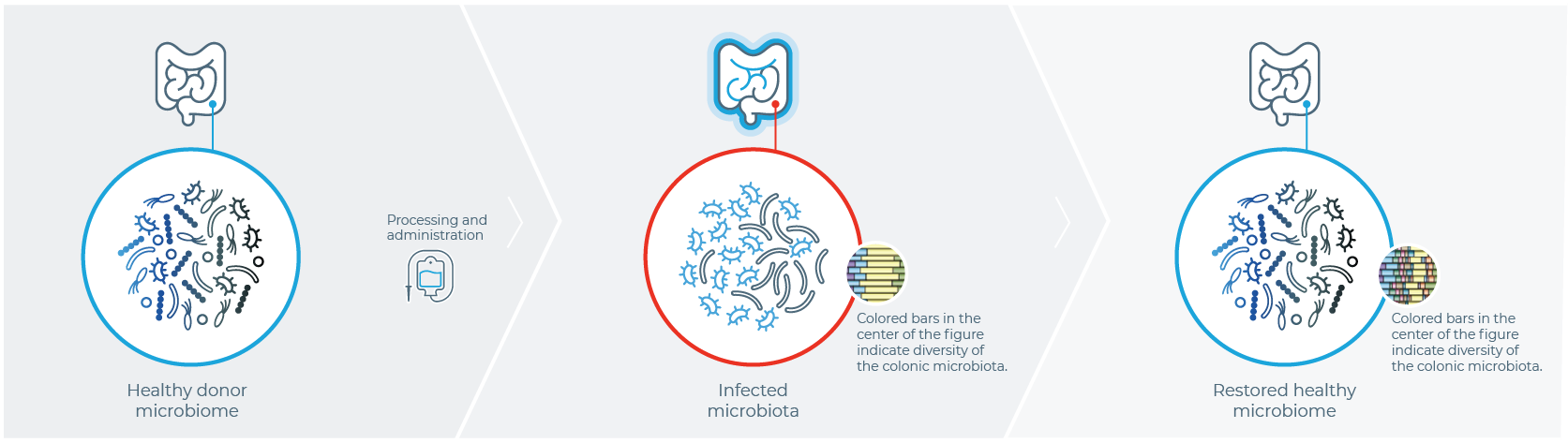
ONE HISTORIC APPROACH TO MICROBIOME RESTORATION
IS FECAL MICROBIOTA TRANSPLANTATION (FMT)1
Healthy Donor
Stool is harvested from a healthy donor
PATIENT WITH rCDI
Fecal sample is transplanted into the patient with rCDI
After FMT
The organisms from the donor sample restore a healthy gut microbiome in the patient
References: 1. American Gastroenterological Association. https//www.gastro.org/practice-guidance/gi-patient-center/topic/fecal-microbiota-transplantation-fmt. Accessed August 31, 2021. 2. Tariq R, et al. Clin Infect Dis. 2019;68(8):1351-1358. 3. Bafeta A, et al. Ann Intern Med. 2017;167(1)34-39. 4. Joseph J, et al. Clin Transi Sci. 2019;12(3)206-208. 5. Guh AY, et al. N Engl J Med. 2020;382(14):1320-1330. 6. McDonald LC, et al. Clin Infect Dis. 2018;66:el-e48.
Limitations of Current Therapeutic Options
SEVERAL LIMITATIONS ARE FOUND ACROSS CLINICAL STUDIES,
DIAGNOSIS, AND TREATMENT
THERE IS VARIABILITY ACROSS CLINICAL TRIALS
Cure rates were lower in randomized controlled trials than in open-label studies (67.7% vs 82.7%, respectively; P<.001).2
This inconsistency is due to considerable heterogeneity among randomized controlled trials, with marked differences in study structure, control groups, fecal transplant materials, and outcome assessments.2,3
LACK OF
STANDARDIZATION
Likewise, the lack of product standardization and administration methods has created a situation where a regulated, safe, and effective product is critically needed.4
PATIENTS ENROLLED IN CLINICAL TRIALS MAY NOT BE A TRUE REFLECTION OF PATIENTS SEEN IN THE REAL WORLD
Strict inclusion and exclusion criteria in randomized controlled trials lead to inclusion of a small portion of patients from daily clinical practice, limiting generalizability of results to patients seen in clinical practice.2
CDI TESTING IS INCONSISTENT IN CLINICAL PRACTICE
There is no gold standard: sensitivity and specificity of current toxin tests for CDI are highly variable. Moreover, in the US, there is no consensus on best diagnostic testing for CDI.5,6
OUR
COMMITMENT
Ferring is committed to exploring the potential of a microbiome-based therapeutic that is studied in a comprehensive population reflective of routine clinical practice for the treatment of recurrent C. difficile infection.
References: 1. American Gastroenterological Association. https//www.gastro.org/practice-guidance/gi-patient-center/topic/fecal-microbiota-transplantation-fmt. Accessed August 31, 2021. 2. Tariq R, et al. Clin Infect Dis. 2019;68(8):1351-1358. 3. Bafeta A, et al. Ann Intern Med. 2017;167(1)34-39. 4. Joseph J, et al. Clin Transi Sci. 2019;12(3)206-208. 5. Guh AY, et al. N Engl J Med. 2020;382(14):1320-1330. 6. McDonald LC, et al. Clin Infect Dis. 2018;66:el-e48.
RESTORE THE GUT MICROBIOME
RESTORE HOPE IN PATIENTS WITH RECURRENT C. diff INFECTION
Further research is essential to ensure availability of a safe, effective, and standardized microbiota-based therapeutic that can help—along with antibiotic treatment—restore the microbiome and break the vicious cycle of recurrent C. diff infection.








References: 1. American Gastroenterological Association. https//www.gastro.org/practice-guidance/gi-patient-center/topic/fecal-microbiota-transplantation-fmt. Accessed August 31, 2021. 2. Tariq R, et al. Clin Infect Dis. 2019;68(8):1351-1358. 3. Bafeta A, et al. Ann Intern Med. 2017;167(1)34-39. 4. Joseph J, et al. Clin Transi Sci. 2019;12(3)206-208. 5. Guh AY, et al. N Engl J Med. 2020;382(14):1320-1330. 6. McDonald LC, et al. Clin Infect Dis. 2018;66:el-e48.